Parkinson's disease commonly manifests with a Riboflavin deficiency
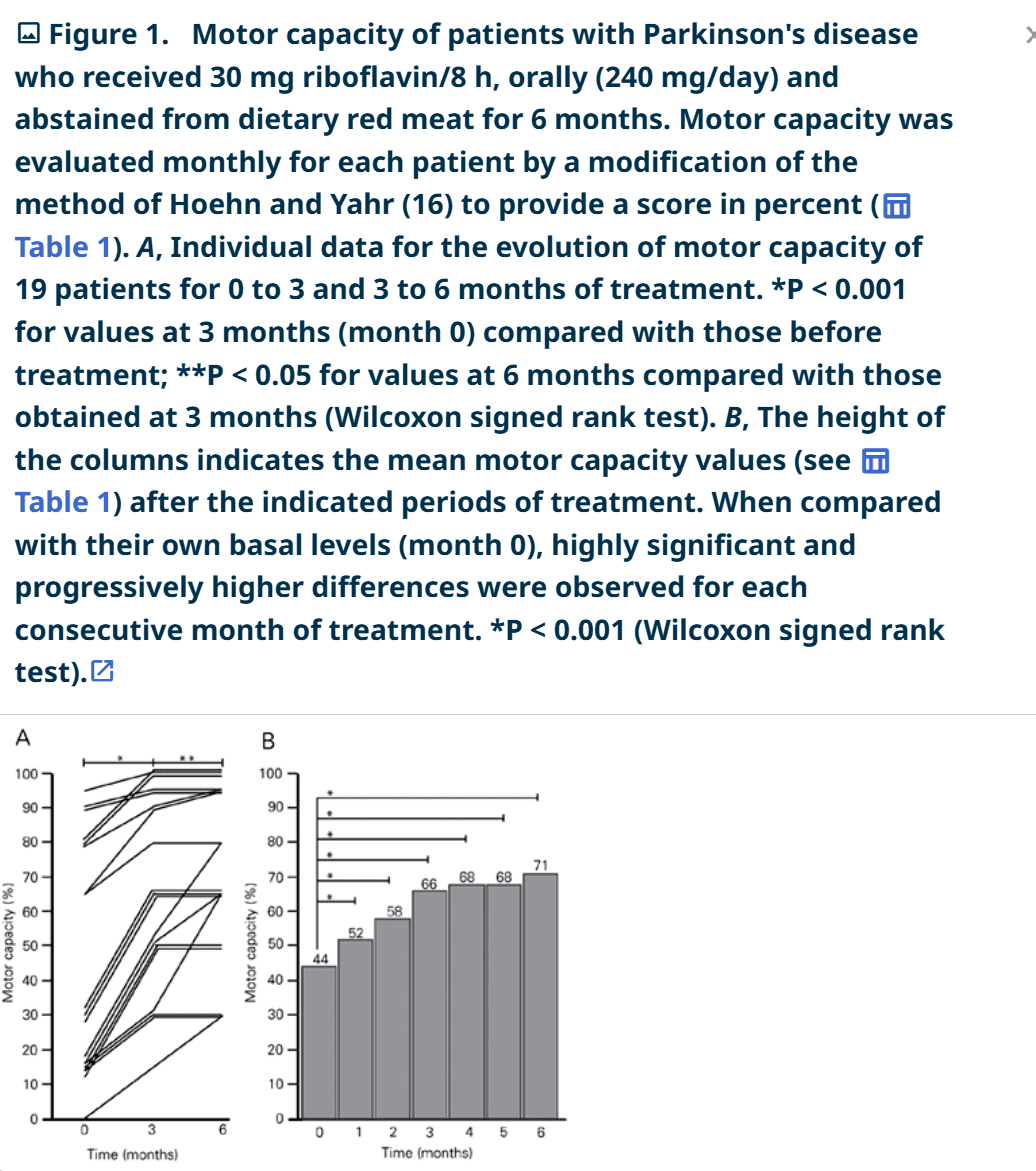
Bottom line up front: After Riboflavin supplementation of 30mg 3x/day (90mg/day) and a no-red-meat-diet, "The mean motor capacity of a group of 19 PD patients showed a progressive 50% recovery over a period of only 3 months - a most surprisingly high and fast improvement, considering that about 60% of nigral neurons have already been lost at the onset of manifestations of PD"
This paper is fantastic! This is a brilliant and simple 23year-old study out of Brazil. The discussion is pure gold (scroll all the way down!) There's so much to unpack here so I'll try and summarize it:
- 1) A large subset of Parkinson's patients have a Riboflavin deficiency.
- 2) They fall into the 10-15% of the general population who have a Riboflavin absorption/utilization deficiency (two enzymes. genetic).
- 3) Riboflavin is a crucial cofactor in digesting iron-rich foods, and replenishing Glutathione. Deficiency leads to glutathione depletion and iron-overload.
- 4) No Red meat: Iron-overload exacerbates the oxidative stress and inflammation throughout the body, including the brain. The study excluded red meat from the diet because red meat contains "hemin", an iron-containing molecule that must be metabolized using Riboflavin. Normally, red meat itself contains adequate Riboflavin, but these people have an inability to utilize it, and instead suffer from an excess of hemin and free iron, which "rusts" and ruins the body. Hemin is considered "neurotoxic." Iron also potentiates the toxicity of mercury, a known culprit in this disease model.
- 5) Glutathione is a critical antioxidant / detoxifier, and depletion leads to mitochondrial dysfunction and disease, including Parkinson's.
- 6) Supplementing Riboflavin allows the body to metabolize and sequester Iron properly, replenishes the body's stores of glutathione (at least partially), and reverses the condition.
- 7) Within 6 months, motor function increased from an average 44% to 77%. In some mild cases, motor function improved to 100%. No negative side effects except the expected yellow urine. No loss of benefit if they skipped a few days (cumulative effect).
My notes:
One other aspect of Parkinson's, and many other neurodegenerative diseases, is heavy metal (and solvent, like toluene) toxicity. Iron potentiates and multiplies the toxic effect of Mercury. Mercury causes glutathione depletion! Any mercury exposure is exacerbated by the Riboflavin deficiency. Glutathione becomes the rate-limiter, and by supplementing glutathione we could also reduce symptoms, but supplementing glutathione is not easy. Before I knew about Riboflavin being so darn effective, I did know that glutathione could have incredible benefit. I'll never forget a patient that came to the SCNM student clinic once per month for a Glutathione IV "push". I would sit beside him and gently push a 100mL syringe into his veins over the course of about an hour. (Glutathione injections can "burn" if they go too fast.) The patient was a former executive with IntelCorp, and had a decades of exposures to metals, acids, and solvents that are used in computer chip-making. His Parkinson's symptoms went completely silent after the glutathione push and he only needed to come once a month to keep it at bay. Glutathione is digested by the gut, and so it can't be given orally. It can be inhaled/nebulized. I could get into that in another blog. For oral supplementation, we suggest "Augmented NAC," which is a name-brand formula of N-Acetyl-Cysteine. NAC is the rate-limiting amino acid used in the formation of glutathione in the body. It's a sulphur-containing amino acid...more on Sulphur later! Sulphur is a big part of detoxification (as well as building collagen, but that's beside the point).
Lab tests: A blood test for Riboflavin status is available through most major labs like Quest.
Riboflavin supplements: Some riboflavin supplements come in a "phosphorylated" form. In this case if would be denoted as R5P or Riboflavin-5-phosphate. We're led to believe that this form is more bioavailable, and skips a step our body would have to do, and saves the energy required to do it. If you don't see R5P, I'm sure the generic form would be helpful.
Why yellow urine?: Riboflavin is water soluble, and yellow-colored. The word "flavin" means "yellow." That simple. There's no harm done to the kidneys on it's way out of the body.
PAPER BELOW: https://www.scielo.br/j/bjmbr/a/BM4WLJBtjxF8Cx3wFsjFhKb/?lang=enHigh doses of riboflavin and the elimination of dietary red meat promote the recovery of some motor functions in Parkinson's disease patients
Abstract
Abnormal riboflavin status in the absence of a dietary deficiency was detected in 31 consecutive outpatients with Parkinson's disease (PD), while the classical determinants of homocysteine levels (B6, folic acid, and B12) were usually within normal limits. In contrast, only 3 of 10 consecutive outpatients with dementia without previous stroke had abnormal riboflavin status. The data for 12 patients who did not complete 6 months of therapy or did not comply with the proposed treatment paradigm were excluded from analysis. Nineteen PD patients (8 males and 11 females, mean age ± SD = 66.2 ± 8.6 years; 3, 3, 2, 5, and 6 patients in Hoehn and Yahr stages I to V) received riboflavin orally (30 mg every 8 h) plus their usual symptomatic medications and all red meat was eliminated from their diet. After 1 month the riboflavin status of the patients was normalized from 106.4 ± 34.9 to 179.2 ± 23 ng/ml (N = 9). Motor capacity was measured by a modification of the scoring system of Hoehn and Yahr, which reports motor capacity as percent. All 19 patients who completed 6 months of treatment showed improved motor capacity during the first three months and most reached a plateau while 5/19 continued to improve in the 3- to 6-month interval. Their average motor capacity increased from 44 to 71% after 6 months, increasing significantly every month compared with their own pretreatment status (P < 0.001, Wilcoxon signed rank test). Discontinuation of riboflavin for several days did not impair motor capacity and yellowish urine was the only side effect observed. The data show that the proposed treatment improves the clinical condition of PD patients. Riboflavin-sensitive mechanisms involved in PD may include glutathione depletion, cumulative mitochondrial DNA mutations, disturbed mitochondrial protein complexes, and abnormal iron metabolism. More studies are required to identify the mechanisms involved.
Parkinson's disease; Riboflavin; Flavin-adenine dinucleotide; Glutathione; Iron; Hemin
Summary of Parkinson's Motor Capacity Scoring System:
Discussion (is pure gold!)
This study demonstrated a progressive and marked improvement of motor capacity in consecutively evaluated patients with sporadic PD who started with below normal laboratory indexes of riboflavin and who eliminated red meat from their diets while receiving high multiple daily doses of riboflavin over a period of 6 months while taking their usual symptomatic medications. The mean motor capacity of a group of 19 PD patients showed a progressive 50% recovery over a period of only 3 months - a most surprisingly high and fast improvement, considering that about 60% of nigral neurons have already been lost at the onset of manifestations of PD (15).
The initial riboflavin status was low in all 31 consecutively evaluated PD individuals, and significantly lower in PD patients compared with those with another neurodegenerative disease also associated with hyperhomocystinemia (DwoSt), suggesting that abnormal riboflavin status may be a specific feature of PD rather than a minor metabolic contributor to the degeneration of nigral neurons. Taken together with the rapid and profound neurological improvement associated with normalization of riboflavin status, this observation suggests that altered riboflavin status may be a cause of neurodegeneration in PD.
Although urinary excretion of riboflavin peaks within 1-2 h and returns to baseline within 5-6 h after a large oral dose (3), the benefit achieved did not vanish in four PD patients over a therapeutic interval of up to 7 days. This observation suggests the occurrence of steady plastic changes rather than a pharmacological effect of high-dose riboflavin treatment to account for the improved motor capacity shown in Figure 1. The steady build-up of the motor recovery observed during the first 3 months of treatment suggests that this treatment paradigm may inactivate fundamental neurodegenerative mechanisms (e.g., glutathione depletion, considered to be an early key event in the pathogenesis of PD (23,24)), possibly allowing regenerative plastic phenomena to occur.
The importance of the elimination of dietary red meat for the results reported here is not known. The content of vitamin B2 in meat in general is considerable (about 0.2 mg/100 g), and diverse cooking procedures cause only minor (7-18%) loss of this micronutrient (25). The daily requirement for individuals above the age of 14 years is £1.3 mg/day. Therefore, if the PD patients had a normal absorptive capacity for vitamin B2, their large ingestion of red meat (up to 700 g/day), associated with milk, rice and beans, fruits and vegetables, should have provided a normal riboflavin status. In contrast, 31 consecutive PD patients had laboratory evidence for riboflavin deficiency (Table 2) suggesting that patients with sporadic PD belong to the subset of the general population (10-15%) (3) that may express a flavokinase with low affinity for vitamin B2, leading to a decreased absorption.
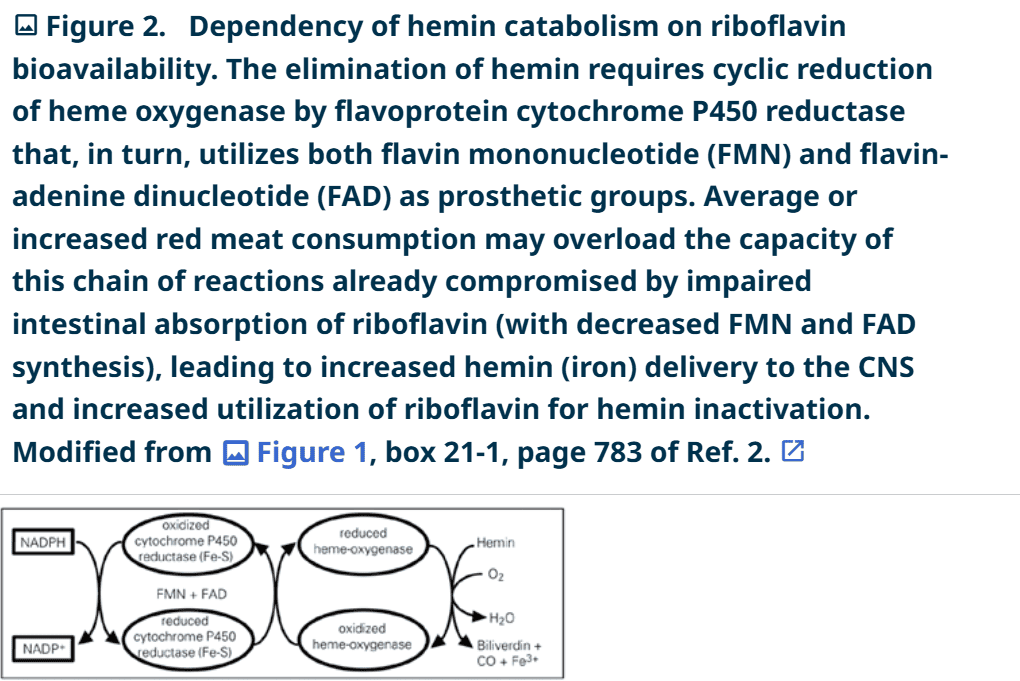
However, the digestion of red meat releases hemin, a highly diffusible toxin that, when not properly inactivated, increases intracellular iron concentrations and enhances hydroxyl radical production (Fenton reaction). Most of the absorbed hemin is destroyed by the enzyme heme oxygenase (HO) in the digestive tract and liver (26). Because HO is oxidized during the catabolization of hemin to biliverdin, the HO molecules must be reduced through the coordinated activity of the flavoenzyme cytochrome P450 reductase for continued hemin inactivation (Figure 2) (27). Cytochrome P450 reductase is particularly sensitive to riboflavin deficiency because it requires both FMN and FAD as prosthetic groups (28). It is possible that individuals with decreased absorption of vitamin B2 may not completely inactivate high dietary levels of hemin, allowing this neurotoxic compound to reach the brain cells. Consistently, the staining for HO-1 isozyme is increased in astrocytes and reacts with neuronal Lewy bodies in the nigra of PD patients, suggesting that its overexpression may contribute to the pathological iron deposition and mitochondrial damage in PD (29). By binding glutathione (30) hemin may further decrease glutathione levels in the brains of PD patients through a direct mechanism.
Because humans lack efficient iron excretory mechanisms, iron excess is dealt with by increasing the synthesis of the iron-storage protein ferritin (31). Disturbed systemic (32) and brain (33) iron metabolism has been reported in PD, suggesting that a selective decrease in the levels of ferritin may result in an increase in intracellular free iron, thereby enhancing free radical production (34). Indeed, vitamin B2 deficiency in rodents is associated with low circulating iron concentrations, increased iron turnover and excretion into the intestinal lumen, which may occur in response to impaired ferritin synthesis (35,36). Therefore, the consistent finding of an abnormal riboflavin status in PD, as reported here, may help to explain the disturbed iron metabolism found in PD patients, with the underlying mechanisms possibly involving impaired hemin catabolism and reduced ferritin synthesis. Interestingly, the highest world prevalence of PD is found among the inhabitants of Buenos Aires (37), where the consumption of red meat is traditionally high. Similarly, the identification of high dietary animal fat as a risk factor for PD (37) may actually reflect a role of high dietary hemin in PD pathology.
Moreover, because FAD is required in the two alternative pathways of deoxynucleotide synthesis (2), DNA repair and replication are expected to be disturbed upon decreased bioavailability of riboflavin, and abnormal riboflavin status may also explain the cumulative mitochondrial DNA mutations reported in PD (38).
The present results with 19 PD patients who showed a significant improvement in motor function after treatment with riboflavin and the elimination of red meat from the diet suggest that an abnormal riboflavin status, possibly due to flavokinase deficiency, may be an essential requirement for triggering and sustaining the degeneration of dopaminergic neurons in PD. As a result of the reduced B2 bioavailability, ATP production is selectively preserved, while the less critical FAD- or FMN-dependent metabolic pathways are impaired (4). Consequently, free iron concentrations in the cytosol increase as a result of impaired ferritin synthesis and/or reduced hemin catabolism associated with hydrogen peroxide accumulation due to glutathione depletion, thereby triggering the Fenton reaction and ultimately leading to the selective formation of the potent neurotoxin 6(OH)DA in dopaminergic neurons.
Current concepts about the cause of sporadic PD suggest an inherited predisposition to environmental or endogenous toxic agents (39), and the data presented and reviewed here suggest that flavokinase deficiency should be considered in future research as a promising candidate to account for this inherited predisposition, while dietary factors such as red meat consumption may largely account for the environmental/endogenous toxicity. The administration of high doses of riboflavin combined or not with red meat elimination may be an effective therapeutic paradigm addressing the determinants of PD, capable of providing regression to earlier clinical stages, or even to the nonsymptomatic state without symptomatic drugs for PD (at least in some cases), rather than only disease stabilization or partial symptomatic relief.
Although the relentless progression of PD clearly contrasts with the results of the treatment paradigm reported here, a larger and more prolonged study is certainly required to document the steadiness and the full extent of the ongoing recovery. A scientifically desirable blinded clinical trial with a placebo would necessarily leave known riboflavin-deficient patients untreated for a long period of time, when their neurological disability may progress as a consequence of sustained loss of nigral neurons, possibly rendering the ultimate response to delayed normalization of their riboflavin levels less complete. Therefore, the need for controlled trials should be weighed ethically considering the contrast of the natural history of PD (progress of motor disability to death despite an increase in the efficacy of symptomatic drugs for PD treatment) with the outcome of the vitamin B2 treatment observed in larger and more prolonged studies without controls.